Antibody Validation: Ensuring Specificity and Reliability in Applications
Antibodies are indispensable tools in a variety of scientific applications, from research and diagnostics to therapeutics. However, the effectiveness of antibodies is contingent on their specificity, reliability, and consistency. Antibody validation ensures that the antibodies used in experiments and clinical applications produce accurate and reproducible results. This article explores the importance of antibody validation, the methods used to validate antibodies, and the key factors to consider when assessing antibody quality.
What is Antibody Validation?
Antibody validation refers to the process of confirming that an antibody is specific to its intended target, performs consistently across different applications, and provides reliable results. Validation helps ensure that antibodies are suitable for the intended purpose, whether for use in Western blotting, immunohistochemistry (IHC), flow cytometry, ELISA, or other applications.
Inaccurate or poorly validated antibodies can lead to incorrect conclusions, wasted resources, and misinterpretations of data. Therefore, proper validation is a crucial step in maintaining the integrity of scientific research and clinical diagnostics.
Why is Antibody Validation Important?
- Specificity: The antibody must recognize and bind only to the target antigen. Cross-reactivity with non-target proteins can lead to false-positive results, undermining the reliability of experiments.
- Consistency: A validated antibody should provide consistent and reproducible results across different experimental conditions, laboratories, and sample types.
- Reproducibility: Antibody validation ensures that results obtained with the antibody can be repeated over time, which is essential for scientific rigor and reliability.
- Regulatory Compliance: In clinical applications, such as diagnostics and therapeutics, regulatory bodies require rigorous validation of antibodies to ensure their safety and efficacy.
Methods of Antibody Validation
Several techniques are employed to validate antibodies, each assessing different aspects of antibody performance. Below are the primary methods used for antibody validation:
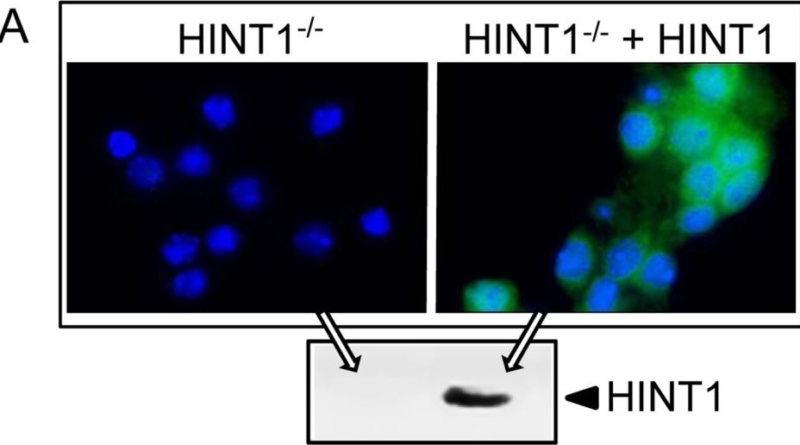
1. Western Blotting
Western blotting is one of the most common methods used to validate the specificity of antibodies. This technique involves separating proteins by size through gel electrophoresis, transferring them to a membrane, and probing with the antibody of interest.
- Validation Aspect: Western blotting confirms the antibody’s ability to detect the target antigen by visualizing a specific band that corresponds to the expected molecular weight of the target protein.
- Control: A positive control (a sample containing the target protein) and a negative control (a sample without the target protein) should be included to ensure specificity.
2. Immunohistochemistry (IHC)
Immunohistochemistry is a technique used to detect the presence of antigens in tissue samples by using antibodies. It provides valuable insights into the tissue-specific localization of the target protein.
- Validation Aspect: IHC validation confirms the antibody’s ability to specifically bind to the antigen in tissue sections. The antibody should only stain the target tissue or cells, not nonspecific sites.
- Control: A negative control (such as a sample without primary antibody) helps ensure that the staining is specific to the target antigen.
3. Flow Cytometry
Flow cytometry is a technique used to detect and analyze the expression of cell surface and intracellular proteins. It can also be used to validate antibodies, particularly in cell-based applications.
- Validation Aspect: Flow cytometry can confirm that the antibody specifically binds to the antigen on the surface or inside the cells. The intensity of fluorescence signals helps determine antibody specificity and affinity.
- Control: A negative control (cells without the antibody) should be used to confirm the absence of nonspecific binding.
4. ELISA (Enzyme-Linked Immunosorbent Assay)
ELISA is a popular method for detecting the presence of specific antigens in solution. It involves immobilizing the antigen on a solid surface and detecting it with an antibody linked to an enzyme that produces a measurable signal.
- Validation Aspect: ELISA confirms the antibody’s ability to specifically bind to the target antigen in solution, often used for both quantitative and qualitative analysis.
- Control: Proper positive and negative controls are essential to distinguish specific binding from background noise.
5. Knockdown or Knockout Models
In this method, researchers use genetic knockdown or knockout models to assess antibody specificity. Knockdown refers to reducing the expression of a target protein, while knockout refers to completely eliminating it from a cell or organism.
- Validation Aspect: If the antibody binds to the target antigen, the protein knockdown or knockout should reduce or eliminate the antibody’s signal. This method confirms that the antibody specifically binds to the target protein.
- Control: A model where the target protein is present should show strong antibody binding.
6. Peptide Blocking
Peptide blocking involves pre-incubating the antibody with a synthetic peptide that corresponds to the target protein’s epitope. If the antibody is specific to that epitope, the presence of the peptide will block the antibody’s binding to the antigen.
- Validation Aspect: A reduction in antibody binding after peptide incubation confirms that the antibody is binding to the specific epitope.
- Control: A peptide that does not correspond to the target epitope can be used as a control to ensure the blocking is specific.
7. Mass Spectrometry
Mass spectrometry can be used to identify the target antigen that binds to the antibody. This technique allows for the precise identification of the proteins recognized by the antibody, providing direct confirmation of specificity.
- Validation Aspect: Mass spectrometry provides an accurate and unbiased method for identifying the binding partners of an antibody.
- Control: A protein sample without the antibody or with an irrelevant antibody should not show any binding.
Key Factors to Consider When Validating Antibodies
Several factors must be taken into account when validating antibodies to ensure their specificity and reliability for research and clinical applications:
1. Antibody Affinity and Specificity
Antibodies should have high affinity and specificity for their target antigen. High-affinity antibodies bind tightly to the antigen, while specificity ensures that the antibody does not cross-react with other proteins.
2. Consistency Across Applications
An antibody validated for use in one application, such as Western blotting, should ideally work consistently in other applications (e.g., IHC, flow cytometry) if the target antigen remains the same. Consistency across different experiments and laboratory conditions is critical.
3. Cross-Reactivity
Cross-reactivity is a common problem where an antibody binds to unintended antigens. This issue is minimized through careful screening and validation. It’s essential to test antibodies in multiple assays to ensure they do not bind to off-target proteins.
4. Batch-to-Batch Consistency
Antibody production batches can vary, leading to inconsistencies in experimental results. Reliable antibody suppliers often provide data on batch-to-batch consistency, and users should test each new batch to ensure it performs as expected.
5. Regulatory Compliance
For clinical applications, such as diagnostic tests or therapeutic antibodies, validation must comply with regulatory standards. Antibodies used for FDA-approved diagnostics or therapeutic purposes require validation to meet stringent quality and safety standards.
Conclusion
Antibody validation is a critical step in ensuring that antibodies are specific, reliable, and reproducible for a wide range of applications. Through methods such as Western blotting, immunohistochemistry, flow cytometry, and peptide blocking, researchers can confirm the antibody’s performance and its suitability for their experiments. By carefully considering factors like affinity, specificity, and consistency, scientists can ensure that their antibodies yield accurate, high-quality results, ultimately advancing research and improving diagnostic and therapeutic outcomes.